CBSE Class 8 Science Chapter 14 Revision Notes
Chapter 14: Chemical Effects of Electric Current Revision Notes
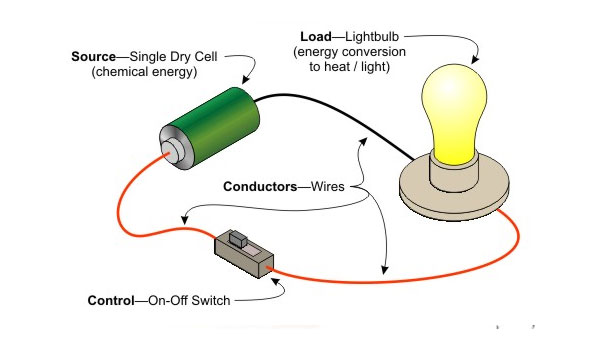
- Good conductors of electricity are materials that permit electric currents to pass through them easily.
- Poor conductors do not allow this flow.
- Copper and aluminium are good electrical conductors.
- Wood, plastic, and rubber are poor electrical conductors.
-
Some liquids are good conductors as well.
-
Purified or distilled water is a poor conductor of electricity.
-
A few contaminants (salts and minerals) make water a good conductor because it can carry electricity. Example: common salt dissolved in water.
-
Solutions of acids, bases, and salts comprise most liquids that conduct electricity.
-
Chemical reactions occur when an electric current passes through a conducting solution. The following are some of the chemical effects of electric current:
- The production of gas bubbles on the electrodes
- Metal deposition on electrodes
- Colour changes in solutions
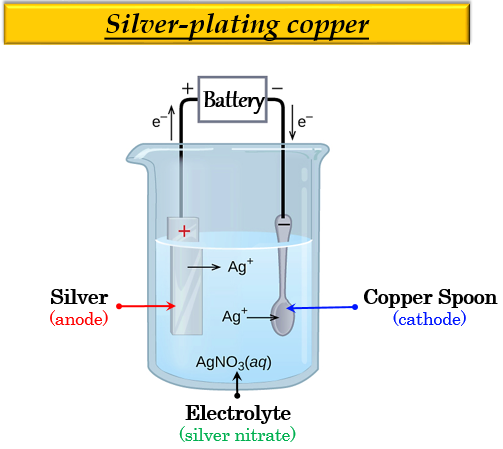
ELECTROPLATING:
Electroplating is depositing a layer of any desired metal on another material using electricity.
- Electroplating is accomplished by immersing two electrodes in the electrolyte and connecting them to a circuit powered by a battery.
- The electrodes (one copper electrode and a brass electrode to be plated) and electrolyte (copper salt solution) are carefully selected.
- When electricity passes through the circuit they create, the electrolyte splits.
- Some of the metal atoms are deposited in a thin layer on top of one of the electrodes, causing it to become electroplated.
ELECTROPLATING USES.
(i) Bath taps and automobile bumpers are plated with chromium to give them a bright, appealing appearance while resisting scratches and wear.
(ii) Silver and gold plating is used on jewellery.
(iii) Tin cans are created by electroplating less reactive tin onto iron and used to store food.
(iv) To prevent iron in vehicles and bridges from rusting, they are frequently covered with zinc.
Source: Chapter-14.pmd (ncert.nic.in)
]]>