CBSE Class 12 Chemistry Chapter 15 Revision Notes
Chapter 15: Polymers Revision Notes
- A polymer is a large molecule with a high molecular mass that is made up of many small molecules called monomers that are repeatedly bonded together.
- Polymerisation is the process by which monomers are converted into polymers.
- Polymers are also known as macromolecules because they are single large molecules.
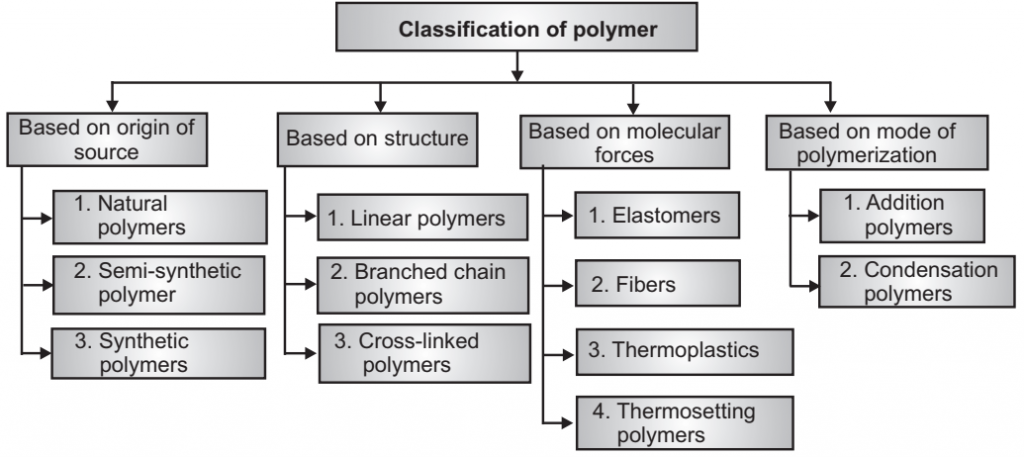
Classification of Polymers
Polymerisation Reactions
- Addition polymerisation, also known as chain growth polymerisation, is a polymerization process in which monomers with one or more double bonds are added in a chain fashion in the presence of an initiator to form a polymer. The free radical mechanism is in charge of it.
- Step growth polymerisation or condensation polymerisation: It happens when monomers condense in a stepwise manner, removing water or other small molecules along the way.
- Copolymerisation: A copolymer is formed when a mixture of more than one monomeric species polymerizes. A copolymer is a polymeric chain that contains multiple units of each monomer. Styrene and methacrylate, for example, form a copolymer.
- Cis 1,4-polyisoprene is a type of natural rubber. It’s an isoprene 1,4-polymer with a linear structure. Rubber latex, which is a colloidal suspension of rubber in water, is used to make it.
- Vulcanisation of rubber is the process of heating a mixture of raw rubber and sulfur between 373 and 415 degrees Celsius. Adding additives like ZnO to the vulcanisation process speeds it up.
- Biodegradable polymers: PHBV and Nylon 2- Nylon-6 were created to reduce the environmental risks associated with synthetic polymeric waste.