CBSE Class 11 Biology Chapter 9 Revision Notes Part 1
Chapter 9: Biomolecules Revision Notes Part 1
Multiple Choice Questions
-
Lysine is an example of a polar but uncharged amino acid called ___________
-
How can we make an amine from an amino acid?__________
-
When converting a disaccharide to monosaccharides, which bond is hydrolyzed? _________
-
Starch is composed of two polysaccharides which are ____________
- Biomolecules are organic molecules produced by living organisms, and they help perform important functions and act as building blocks of life.
- Their composition consists of carbon, hydrogen, nitrogen, phosphorus, sulphur, and oxygen.
- These biomolecules are divided into micro molecules like amino acids, fatty acids, nitrogenous bases, sugars, etc., and macromolecules like carbohydrates, lipids, proteins, and nucleic acids.
What is a biomolecule?
- A biomolecule is also known as a biological molecule.
- They are the numerous substances produced by living organisms in their cells.
- The 4 major types of biomolecules are carbohydrates, nucleic acids, proteins, and lipids.
- All biomolecules share common fundamental relationships with their function and structures.
- These relationships are influenced by various factors such as the environment, the temperature it has to adapt to, humidity conditions, etc.
- For example, the lipids are hydrophobic – water phobic. Hence, many immediately arrange themselves so that the hydrophobic ends of these lipids are protected from coming in contact with water. This arrangement emerges as lipid bilayers.
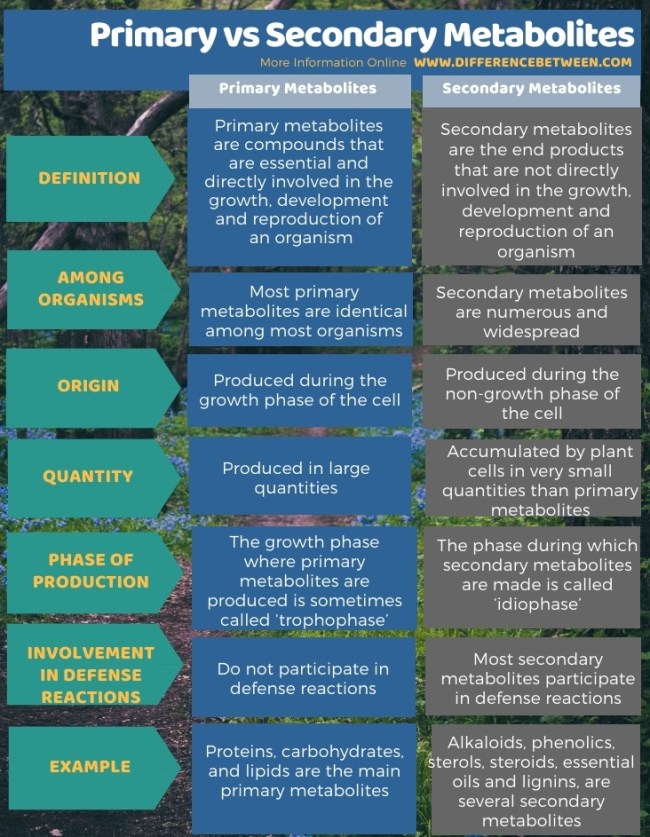
Primary and secondary metabolites
- If one were to make a list of biomolecules, such a list would have thousands of organic compounds including amino acids, sugars, etc.
- For various reasons we can call these biomolecules as ‘metabolites’.
- In animal tissues, we notice the presence of all such categories of compounds.
- These are called primary metabolites.
- Some cells have many compounds other than the primary metabolites, e.g. alkaloids, flavonoids, rubber, essential oils, antibiotics, coloured pigments, scents, gums, spices.
- These are called secondary metabolites.
- While primary metabolites have **identifiable functions **and play important roles in the physiological processes.
Functions of biomolecules
The functions of biomolecules are as follows;
-
They are important as they aid organisms to grow, sustain themselves, and reproduce.
-
They involve themselves in building organisms from small single cells to complex living beings like humans.
-
They help store energy, as in the case of carbohydrates.
-
They catalyze biochemical reactions.
-
They help transmit RNA and DNA genetic codes and store them.
Types of Micro molecules
1. Amino Acids
- Amino acids are smaller acids that form the building blocks of proteins.
- In chemical forms, each amino acid is made up of a carboxylic acid, hydrogen atom, a variable R, and an amine group attached to carbon atoms known as alpha carbons.
- Based on the nature of the R Groups, approximately 20 amino acid variants are present, and these acids are substituted methane groups.
- The amino acids can also exist in zwitterionic forms as they change according to the solution’s pH values.

Source: Amino acid structure
Certain amino acids are essential as they are required in our diets. There are 9 such ones:
- Leucine
- Valine
- Lysine
- Histidine
- Threonine
- Phenylalanine
- Isoleucine
- Tryptophan
- Methionine
There are also nonessential amino acids that are formed inside the body. The acids can be basic, neutral as well as acidic in nature. They include:
- Alanine
- Aspartic
- glutamic acid
- Serine
- Tyrosine
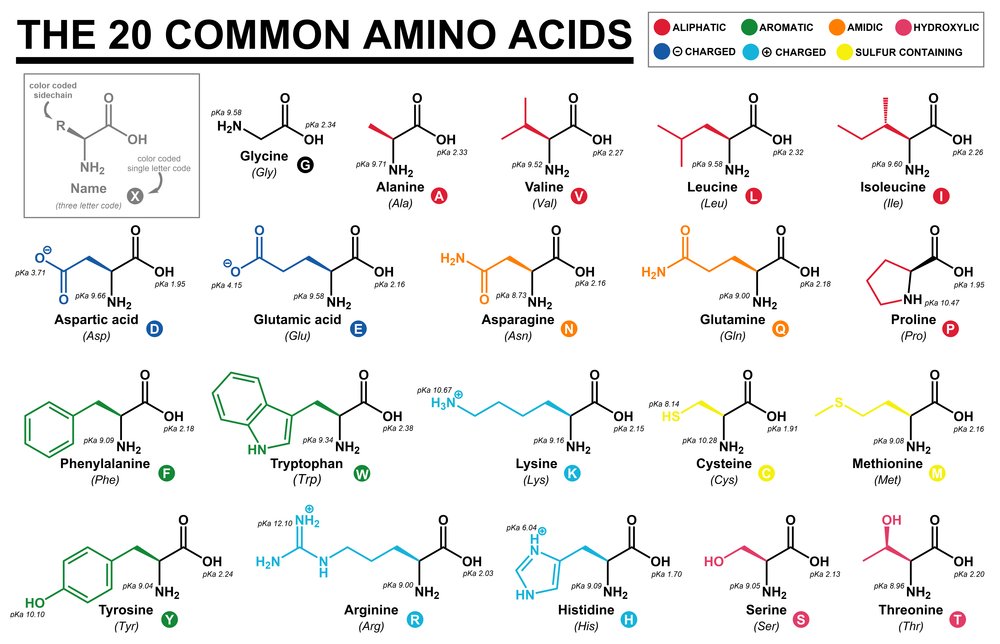
Source: Common Amino Acids
2. Fatty acids
- They are generally carboxylic acids with aliphatic chains.
- They can be saturated containing single carbon bonds or unsaturated containing multiple carbon bonds.
- These acids are important constituents of fats like triglycerides which are groups of 3 fatty acid molecules.
- These triglyceride fats are one of the biomolecules that help store chemical energy which fuels the process of metabolism like muscular contractions.
- They are also an important structural component of the biological membranes of organelles and cells.
- They are also called the building blocks of fat in our bodies. Some popular examples of fatty acids are cholesterol, oils, steroids, and fats.
3. Nitrogenous bases
- A nitrogenous base is an organic molecule that primarily contains nitrogen.
- These bases are molecules having heterocyclic structures, and they act as bases in chemical reactions.
- There are 2 types which are purines and pyrimidines.
- Purine bases are of 2 types: Adenine and Guanine.
- Pyrimidine bases are of 3 types: Thymine, Cytosine, and Uracil.
4. Sugars
- These sugars are monosaccharides and polysaccharide molecules that form carbohydrates.
- Monosaccharide molecules are carbohydrates with single sugars, and disaccharide molecules are carbohydrates with 2 or more simple sugar molecules.
- DNA contains deoxyribose sugar molecules as well as RNA contains ribose sugar molecules.
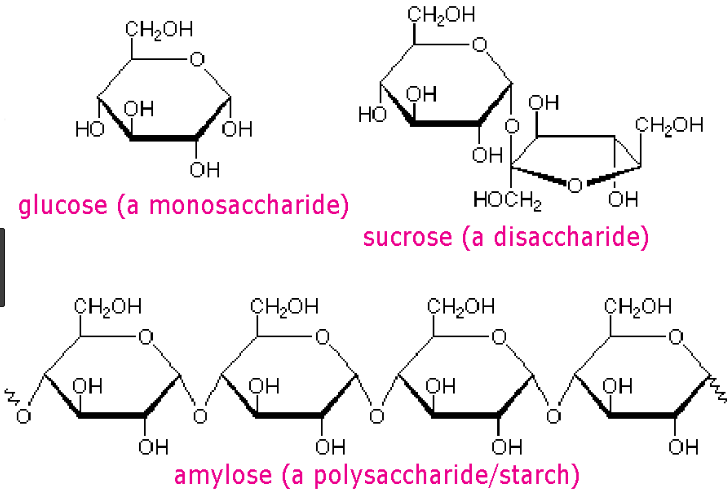
Source: Sugar structure
]]>